Any material has a certain permeability
Any material has a certain permeability. The specific use of different polymers will determine the permeability for oxygen, nitrogen, carbon dioxide, water vapour and other gasses.
The barrier made by these polymers ensures that the gas composition inside a package can be maintained which improves the guarantee period of the packed product. Two different indexes are use to determine this phenomenon: permeability and permeation mass. Permeability is a characteristic of the material and will vary with material thickness or area. Permeation is a property of the finished product and changes with material thickness and structure.
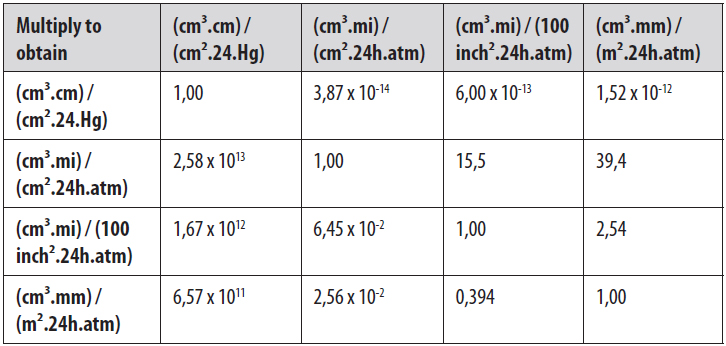
1. GAS PERMEABILITY AND PERMEATION MASS
Gas permeability is usually expressed as gas permeability coefficient (P) and permeation mass (Q). The permeability of an inorganic gas is determined by the solubility (S) and diffusion coefficient (D). The permeability coefficient refers to the amount of gas, as a volume, which penetrates per unit of thickness and area of a specimen over a time period, under constant temperature and pressure difference. The unit is cm³.cm/cm².s.Pa. The permeation mass refers to the gas volume which permeates per area of a specific specimen, with a constant temperature and pressure difference. The unit is cm³/m².d.Pa.
Following formula can be used:
P = Q x d
d = material thickness
Depending on the test standard which is used, several other units may be possible, like cm³/m².d.atm, ml(STP)/m².d, etc.
A conversion table can be useful.
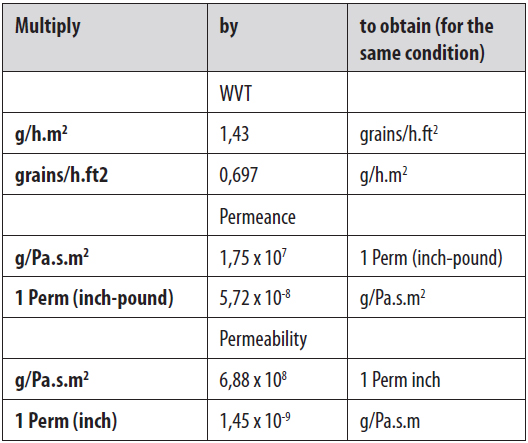
2. WATER VAPOUR PERMEABILITY COEFFICIENT AND TRANSMISSION RATE
Water vapour permeability is expressed as water vapour permeability coefficient and transmission rate. The latter is the most used.
Water vapour permeability coefficient (Pv) refers to water vapour volume that permeates per unit of thickness and area of a specimen over a time period, under specified temperature, relative humidity and vapour pressure difference. The unit is g.cm/cm².s.Pa. Water vapour transmission rate (WVTR) refers to the amount of water that penetrates one square meter of sample with a specific thickness, during 24 hours and under specified temperature, relative humidity and vapour pressure difference. The unit is g/m².24h. As for gas permeability, also different units can be used.

3. THE NEED TO TEST BARRIER PROPERTIES OF A PACKAGE
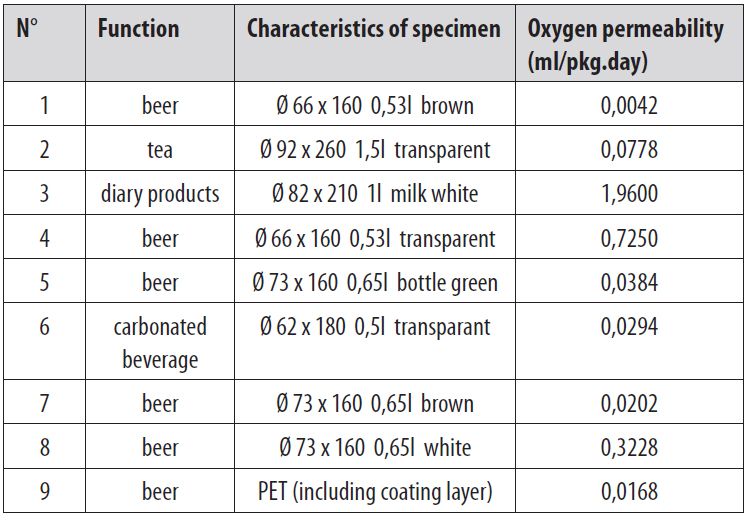
To explain the need to test, an example of a bottle with a cap is taken. In this case, strictly spoken, this test should include three parts. The barrier of the bottle cap, the bottle and the connection between the cap and the bottle. Since barrier properties of the package can directly influence the quality of its inner content, the focus is on how to improve these properties. One of the most important parameters to be tested is the barrier for oxygen. This is because oxygen is the main reason for product deterioration. Also other gasses as nitrogen, carbon dioxide can be of importance. Oxygen permeability is often tested according to the standard ASTM F 1307.The oxygen permeability of the plastic bottles is mainly between 0,02 and 0,4 ml/pkg.day. After adding a special barrier material, the bottles can achieve a oxygen permeability lower than 0,02 ml/pkg.day.
4. HOW TO SELECT A GAS PERMEABILITY TEST METHOD
Differential-pressure method compared to Equal-pressure method
For the differential-pressure method, the vacuum method is most common. In this method, the diffusion of the test gas trough the specimen only occurs in a one direction and in one single medium. This is considered as self-diffusion and can be described with Fick’s law. In the equal-pressure method the most common used method is the sensor method. As both sides of the specimen are at equal pressure there is a diffusion in both ways. This is described as inter-diffusion. Test data have shown that both methods can give different results. Therefore the selection of the right method is of vital importance.
Advantages of the vacuum method
This method has no selectivity for a test gas. Any non-corrosive or non-toxic gas can be tested. Only a small quantity of test gas is needed to perform a test. As there are almost no consumable components in this instrument, this is a rather cheap test method. Impure gasses in the test environment can be completely neglected as the test chamber are evacuated to below 27 Pa before filling again with pure test gas.
Advantages of the sensor method
As a constant and equal pressure is maintained at both sides of the specimen, this method avoids the rupture of a package due to a big pressure difference between inner and outer side. With this method nearly all types of bottles, boxes, bags, … can be tested. This avoids errors caused by estimation made by only testing a sheet of the package. Since nitrogen gas is used as a carrier gas, this method is not applicable to measure the permeability for this gas.
Gas permeability is usually expressed as gas permeability coefficient (P) and permeation mass (Q). The permeability of an inorganic gas is determined by the solubility (S) and diffusion coefficient (D).